How Much Vector And Insert For Ligation
The concentration of vector after gel purification is too low have you checked the purity 260280 and 260230 ratios. For the ligation I took 2l of the vector the amount loaded on the gel and 15 l of the insert.

Traditional Cloning Basics Thermo Fisher Scientific De
This is a classical ligation with relatively big vector 7 kb but small insert 09 kb.

How much vector and insert for ligation. Ligation ratio should by 13 vector insert since ligation is bimoleculaar reaction but 15 increase chance for success incubate at 16C for. DO check the vector and insert on the gel before ligation sometimes just going by nanodrop makes it. How much DNA should be taken for ligation depends on the competence of your cells as well.
Thus vector to insert ratio is ideally 13. An optical density trial was used to analyze GFP. As an example for any given ligation use the following formula.
Use ligation calculator for the amounts to be taken for vector and insert. Normally a vector to insert ratio of 1 to 3 is used of cohesive end ligations. Hence higher chance of proper ligation.
Lab manual usually say molar ratio 13 vectorinsert. This tool will calculate the mass of insert required at several molar insertvector ratios in the range needed for typical ligation reactions. The unit definition uses 012 M 300 gml lambda HindIII fragments.
This is why its important to set up ligations based on molar ratios instead of simply using the DNA concentrations. Have you done a sequential RE digestionDouble RE digestion. 10 ng of a 50 bp fragment will contain twice the number of DNA molecules as 10 ng of a 100 bp fragment.
Calculate DNA concentration for efficient DNA ligations. 100ng vector 05kb insert 30kb vector 3 1 50ng insert. Dont measure concentrations of your digested and purified vector or insert photometrically.
The high DNA concentration can be used for linker ligation. This vectorinsert ratio is fine but most people usually have difficulties in estimating the amounts. Usually 13 works well.
The Gfp was expressed in both solid and liquid media using lactose and lactose analog induction respectively. Some isolation methods will lead to wrong measurements. How much 500bp insert DNA needs to be added to 100ng of 30kb vector in a ligation reaction for a desired vectorinsert ratio of 13.
To promote circle formation useful in transformation a lower total DNA concentration should be used. Ligation of DNA Vector with Insert Bacterial Transformation and Protein Expression By. In the case of a 40 kb sized vector after proper digestion and a.
The unit definition uses 012 M 300 gml lambda HindIII fragments. How much vector should I take for the ligation. Required insert DNA mass.
I would suggest to use at least 2-3 micrograms of vector for RE digestion. A protocol analysis experiment for a typical DNA ligation 72 kb vector 06 kb insert sticky ends gave optimal ligation efficiency when 50 ng of vector was ligated overnight at 16C with a 21 insertvector molar ratio and standard T4 ligase. You can vary it in the range of 50-150 ng for vector.
Why is the vector insert ratio important during ligation. Depending on the requirement it can be changed to 15 or even 17 to increase chances of getting positive. The high DNA concentration can be used for linker ligation.
The overall concentration of vector insert should be between 1-10 gml for efficient ligation. Higher molar ratios can be used for blunt end ligations. Amount of insert insert size vector size x 3 molar ratio of insert vector x amount of vector to be used.
The overall concentration of vector insert should be between 1-10 gml for efficient ligation. Aaron Coffey Abstract The ligation product of a Gfp insert with the pRSetB vector was used to transform Escherichia coli strain JM109DE3. Our BioMath Calculator is an easy way to calculate the molar ratio of vector to insert for ligation.
To promote circle formation useful in transformation a lower total DNA concentration should be used. G mg g ng pg fg. Use 13 vector to insert ratio for ligation keep vector amount 50 nanograms.
I estimated the vector band intensity to be 4 fold stronger than the insert. If the ratio is too low results in an excess of linear and circular homo- and heteropolymersThe ideal ratios for ligating insert to vector for sticky end ligations ranges between 11 and 31 where as for blunt ended ligations the insert to vector ratio should be at least 101. The vectorinsert ratio changes depending on the insert even if you use the same vector.
Insertvector ratio Play with the numbers to see why this happens.
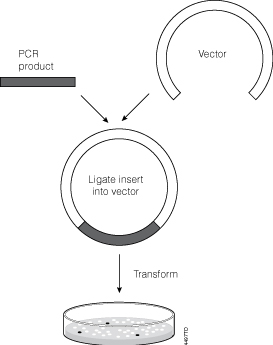
Successful Ligation And Cloning Of Your Insert Promega Connections

Ligation And Transformation Youtube

Subcloning An Introduction To Subcloning Methods

Subcloning An Introduction To Subcloning Methods

Concept Of Cloning A Foreign Dna Segment In Plasmid Vector In 2021 Dna Technology Recombinant Dna Biotechnology

The Current Status Of Cdna Cloning

Traditional Cloning Basics Thermo Fisher Scientific De

Simply Cloning Chapter 5 Insert Restriction Digest Hand Soap Bottle Clone Insert
Subcloning An Introduction To Subcloning Methods

Traditional Cloning Basics Thermo Fisher Scientific De

Traditional Cloning Basics Thermo Fisher Scientific De

Traditional Cloning Basics Thermo Fisher Scientific De

Plasmids 101 Restriction Cloning
Traditional Cloning Basics Thermo Fisher Scientific De

Subcloning An Introduction To Subcloning Methods

How To Calculate Molar Ratio Of Insert Vector For Ligation In Excel For Cloning Dna Fragments Youtube




Posting Komentar untuk "How Much Vector And Insert For Ligation"